12+ Mirikizumab Complete Response Letter
Web In mid-April the FDA issued a complete response letter for mirikizumab related to manufacturing issues but in a release on the letter Lilly noted that the agency. Food and Drug Administration issues Complete Response Letter for dasiglucagon in congenital.

Xipere Triamcinolone Acetonide To Treat Macular Oedema Associated With Uveitis
Web INDIANAPOLIS April 13 2023 PRNewswire -- Eli Lilly and Company NYSE.

. Analysts with Evaluate Vantage. INDIANAPOLIS April 13 2023 PRNewswire -- Eli Lilly and Company NYSE. 14th Apr 2023 Eli Lilly and Company announced the FDA has.
Food and Drug Administration FDA has issued a complete. Web The road to approval was delayed by a Complete Response Letter from the FDA in April 2023 which expressed concerns related to the proposed manufacturing of the agent. LLY announced the US.
Web April 13 2023. 19 2023 PRNewswire -- Eli Lilly and Company NYSE. LLY today announced the US.
Web April 14 2023 at 1133 AM 2 min read. An important regulatory submission from Eli Lilly NYSE. Food and Drug Administration FDA has issued a.
Web INDIANAPOLIS April 13 2023 PRNewswire -- Eli Lilly and Company NYSE. LLY mirikizumab biologic license application BLA for. Web INDIANAPOLIS April 13 2023 PRNewswire -- Eli Lilly and Company NYSE.
Web Complete response letter for mirikizumab to treat ulcerative colitis Read time. Web The regulator has issued a complete response letter. LLY announced the US.
Web The FDA complete response letter disrupts Lillys plan to introduce a first-in-class med and potential blockbuster in ulcerative colitis. Web Get 7 Days Free Sign In Sign In Topics. Web April 14 2023 The Food and Drug Administration FDA has issued a Complete Response Letter to Eli Lilly regarding the Biologics License Application for mirikizumab an.
No concerns related to the clinical data package safety or the medicine label. Web --Eli Lilly and Company announced the US. Web In April the company announced that it had received a complete response letter for mirikizumab for UC as a result of issues FDA identified related to the proposed.
Food and Drug Administration FDA has issued a complete. Web The FDA has issued a complete response letter for Eli Lilly And Cos LLY mirikizumab biologic license application BLA for ulcerative colitis UC. LLY has not been.
Web Surprise setback for mirikizumab as US regulator denies approval. Web The US Food and Drug Administration FDA has issued a Complete Response Letter CRL for mirikizumab a potential treatment for ulcerative colitis citing issues related to. Web Of patients who had responded to the 12-week induction treatment with mirikizumab nearly half achieved clinical remission at the 1-year follow-up after.
Company announcement No. LLY announced the US. The FDA has issued a complete response letter for Eli Lilly And Cos NYSE.
Food and Drug Administration FDA has issued a. LLY announced the US. Food and Drug Administration has issued a complete response letter for the mirikizumab biologic license application for the.
Food and Drug Administration FDA has issued a. Web The FDA issued a complete response letter to Eli Lilly indicating it cannot approve the companys biologic license application seeking approval for mirikizumab as. Eli Lilly executive vice.

How To Request Congratulatory Letters For Eagle Scouts
Fda Rejects Eli Lilly S Inflammatory Bowel Disease Drug Citing Production Plans Medwatch

European Crohn S And Colitis Organisation Ecco Dop09 Mirikizumab Induced Transcriptome Changes In Patient Biopsies At Week 12 Are Maintained Through Week 52 In Patients With Ulcerative Colitis

Efficacy Of A Fourth Dose Of Covid 19 Mrna Vaccine Against Omicron Nejm
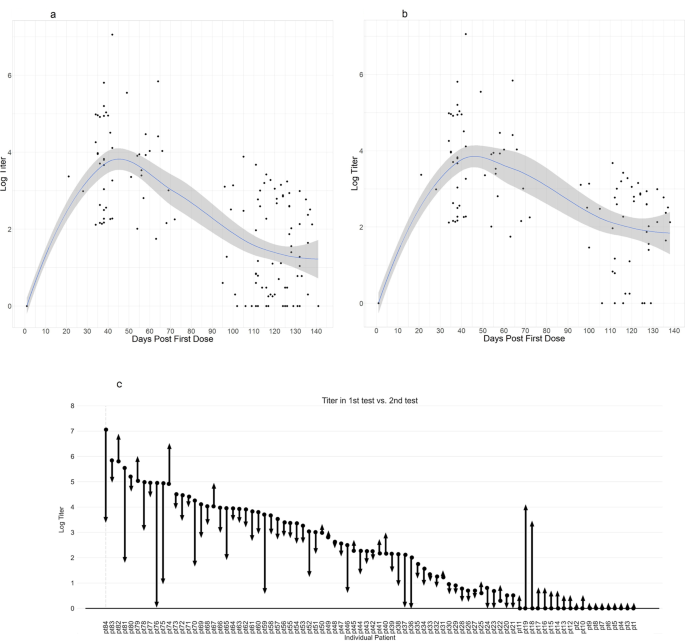
Antibody Persistence 100 Days Following The Second Dose Of Bnt162b Mrna Covid19 Vaccine In Patients With Chronic Lymphocytic Leukemia Leukemia

External Manufacturing Issues Thwart Fda Approval Of Lilly S Lebrikizumab For Dermatitis

Fda Hits Both Lilly And Rentschler With 483s Bioprocess Insiderbioprocess International
Lilly Unlucky Lilly Leads Big Pharma In Us Fda Complete Response Letters Pink Sheet
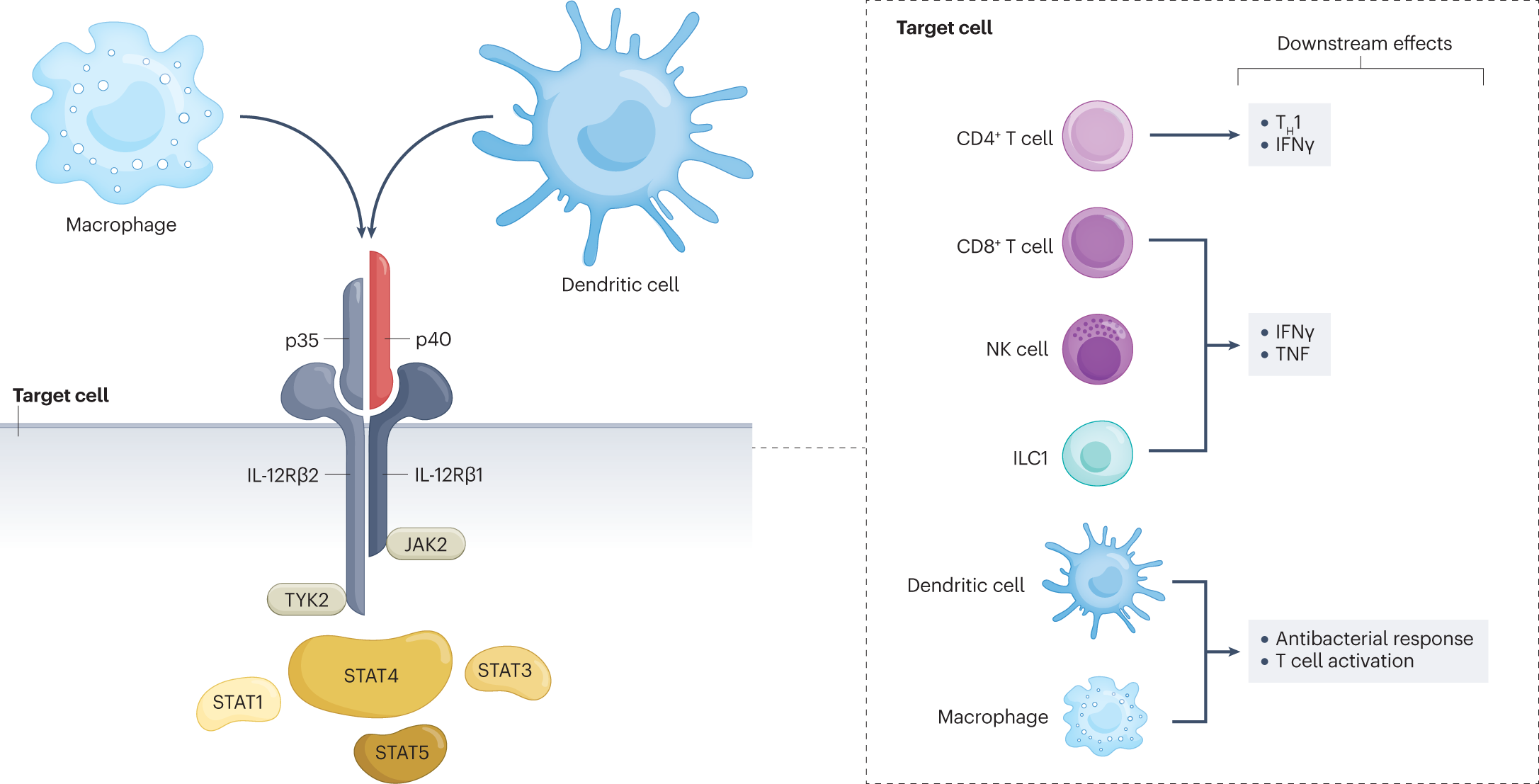
Il 12 And Il 23 Pathway Inhibition In Inflammatory Bowel Disease Nature Reviews Gastroenterology Hepatology

Identification Of Imidazo 1 2 B Pyridazine Derivatives As Potent Selective And Orally Active Tyk2 Jh2 Inhibitors Acs Medicinal Chemistry Letters

Mirikizumab Approval Denied Over Issues Related To Proposed Manufacturing
Manufacturing Issues Delay Lebrikizumab S Entry To The Atopic Dermatitis Market
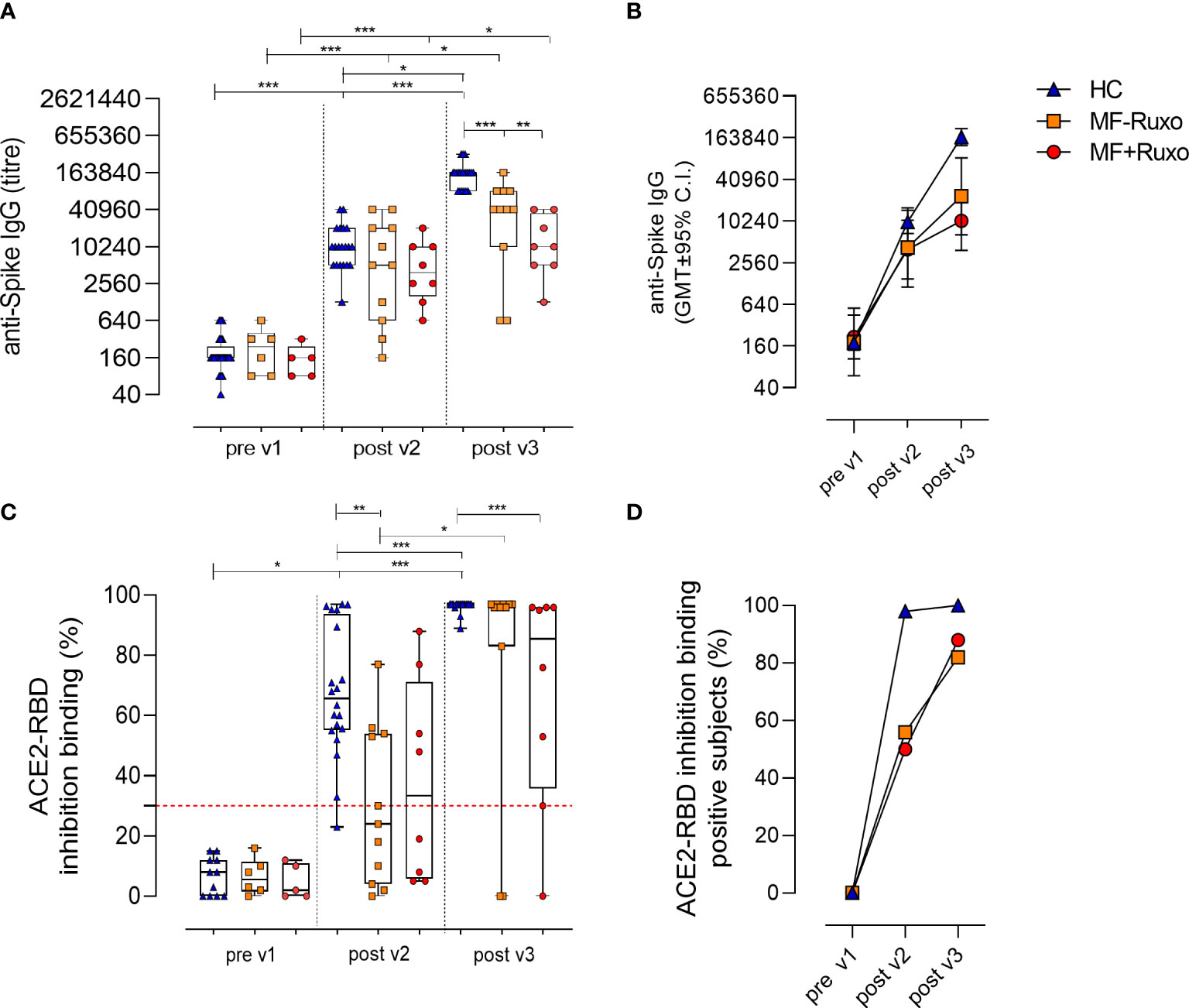
Frontiers The Third Dose Of Mrna Sars Cov 2 Vaccines Enhances The Spike Specific Antibody And Memory B Cell Response In Myelofibrosis Patients

Generation And Characterization Of Mirikizumab A Humanized Monoclonal Antibody Targeting The P19 Subunit Of Il 23 Journal Of Pharmacology And Experimental Therapeutics
Vykipw3erc6f5m

Mirikizumab Has Qol Benefits In Analysis Of Lucent Uc Data

European Crohn S And Colitis Organisation Ecco Dop87 Association Of Histologic Measurement With Endoscopic Outcomes After One Year Of Treatment With Mirikizumab In Patients With Moderate To Severe Crohn S Disease